The Role of Calcium in Preventing Stress Fractures
Bone remodeling resembles an endless construction project where specialized cells work in perfect coordination. Osteoclasts carefully remove aged or damaged sections while osteoblasts deposit fresh calcium-rich matrix. This meticulous process requires precise mineral availability—too little calcium and repairs become shoddy, too much and deposits may form in soft tissues.
The remodeling cycle demonstrates calcium's dual role as both structural element and cellular signal. Ions flowing through microscopic channels direct construction crews to high-stress areas needing reinforcement. This elegant communication system allows bones to strategically strengthen where needed most—a capability that diminishes when calcium signals grow weak.
The Importance of Calcium in Preventing Osteoporosis
Osteoporosis represents the cumulative effect of decades of calcium mismanagement—a condition where bones become so porous they fracture under ordinary stresses. The disease's insidious progression highlights why peak bone mass development during youth proves so critical. Like retirement savings, the calcium deposited before age thirty determines skeletal resilience in later decades when withdrawals inevitably exceed deposits.
Dietary Sources of Calcium
While dairy products dominate calcium discussions, the mineral appears in surprising abundance across the culinary spectrum. Sardines with their edible bones, calcium-set tofu, and fortified plant milks offer alternatives for those avoiding lactose. Even herbs like basil and thyme contribute measurable amounts, proving that diverse eating patterns can support skeletal needs when properly planned.
Calcium Supplements: When and How to Use Them
Supplementation requires careful consideration—the equivalent of taking vitamin pills with a microscope. Different forms (carbonate vs citrate) suit varying digestive conditions, while timing affects absorption efficiency. Medical guidance proves essential, as excessive supplementation risks vascular calcification while insufficient dosing leaves bones vulnerable. The ideal approach combines targeted supplementation with dietary optimization for comprehensive coverage.
Calcium Intake and Stress Fracture Risk
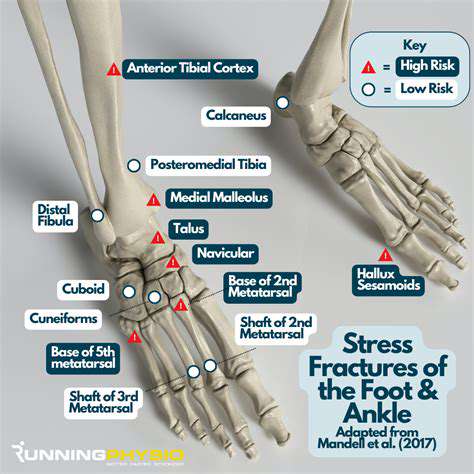
Understanding Stress Fractures
Stress fractures begin as microscopic fissures—the skeletal equivalent of hairline cracks in overloaded aircraft wings. These injuries follow predictable patterns in specific populations: metatarsal fractures in rookie soldiers, tibial stress reactions in distance runners, and vertebral compression in osteoporotic elders. Recognizing these patterns allows for targeted prevention strategies before minor damage progresses to complete fractures.
The injury mechanism reveals bones as living stress monitors. Each impact creates microscopic damage that normally triggers repair processes. When cumulative damage outpaces reconstruction—whether from excessive activity, nutritional shortfalls, or hormonal imbalances—the repair system becomes overwhelmed. This imbalance transforms adaptive remodeling into pathological deterioration, with calcium availability determining how quickly the scales tip toward injury.
The Role of Calcium in Bone Health
Calcium's biological role extends far beyond simple structural support. The mineral participates in electrochemical signaling that coordinates fracture repair and mechanical adaptation. Bones constantly monitor their calcium reserves like engineers checking bridge suspension cables—when levels drop, non-essential repairs get postponed until mineral supplies replenish.
The calcium-bone density relationship follows a dose-response curve with diminishing returns. While severe deficiency guarantees problems, optimal intake provides maximum protective benefit. This explains why population studies show fracture rates plummeting with improved calcium status up to a plateau point. Strategic intake positioning within this protective range makes the difference between resilient and fragile bones.
Calcium Intake and Stress Fracture Prevention
Preventive calcium strategies must account for individual absorption variability—a 300mg dietary dose might yield anywhere from 60-150mg absorbed calcium depending on digestive health and co-consumed nutrients. This variability explains why blanket recommendations often fail, and why personalized approaches combining dietary assessment with targeted supplementation prove most effective.
The synergy between calcium and weight-bearing exercise creates particularly powerful protection. Mechanical loading directs mineral deposition to high-stress areas, while adequate calcium ensures raw materials for reinforcement. This partnership explains why sedentary individuals with good calcium intake still face elevated fracture risk—bones need both the architect's plans (mechanical signals) and the builder's materials (calcium) for optimal strength.
Supplementation and Stress Fracture Prevention
Understanding Stress Fractures
Stress fractures represent overuse injuries with distinct developmental stages. The progression from bone fatigue to complete fracture follows predictable histological changes—first osteoclastic resorption outpacing formation, then microdamage accumulation, finally macroscopic failure. Recognizing these stages allows for intervention before irreversible damage occurs, with calcium status influencing progression speed at each phase.
The Role of Calcium in Bone Health
Calcium's mechanical role parallels rebar in concrete—providing tensile strength to resist bending forces. Without adequate mineral content, bones behave like poorly reinforced structures, developing stress concentrations that propagate cracks. This explains why calcium-deficient individuals often present with unusual fracture patterns—the equivalent of structural failures occurring at load levels that should cause minimal strain.
Calcium Absorption and Vitamin D
The calcium-vitamin D partnership functions like specialized logistics team. While calcium represents the building material, vitamin D operates as both forklift operator and site foreman—enhancing intestinal absorption while directing mineral to appropriate deposition sites. This synergy explains why isolated calcium supplementation often disappoints—without adequate vitamin D cofactors, much of the mineral never reaches its skeletal destination.
Supplementation Strategies for Athletes
Elite athletes require customized calcium protocols accounting for sweat losses, acidic diets, and circadian absorption patterns. Morning loading doses may optimize absorption when vitamin D levels peak, while evening doses could leverage nocturnal bone remodeling cycles. Such precision dosing requires professional guidance to avoid unintended consequences while maximizing protective benefits.
Importance of a Balanced Diet
Whole-food calcium sources provide co-delivered nutrients that enhance utilization—magnesium for crystal formation, vitamin K for matrix proteins, phosphorus for hydroxyapatite formation. This nutritional symphony explains why dairy products outperform isolated supplements in some studies—the complete nutritional matrix supports more efficient bone building than isolated components.
Beyond Calcium: Other Essential Nutrients
The skeletal system's complexity demands broad-spectrum nutritional support. Silicon aids collagen cross-linking, zinc supports enzymatic remodeling, and boron influences mineral metabolism. This nutritional ecosystem approach recognizes bones as living organs requiring diverse inputs—not just calcium monotherapy—for optimal resilience against stress fractures.